IP basics in Life sciences
Last time, we discussed how to obtain a patent, following the process from application to obtaining a patent. I touched on the length of a patent term at the end. Do you remember how long a patent term was? The answer is 20 years from the filing date, and in the case of pharmaceutical patents, the term can be extended by up to 5 years, making the maximum duration 25 years.
So, in this volume, I’d like to talk about patent term extensions for pharmaceuticals.
Vol. 4: What do you mean “patents can be extended”?
– Patent Term Extension: A special system for pharma:
What is a patent term extension?
As mentioned, the patent term is 20 years from the filing date. However, patents that protect certain products—specifically pharmaceuticals, regenerative medicine products, and agrochemicals in Japan—may be extended by up to 5 additional years. As a result, the total patent term may reach up to 25 years from the filing date. This system is referred to as the patent term extension.
Why can the pharmaceutical patent term be extended?
As discussed in Volume 2, the manufacture and sale of pharmaceuticals (specifically, prescription drugs) requires approval from the Ministry of Health, Labor and Welfare (MHLW). This requires that clinical trials be conducted and the resulting data submitted with a new drug application. Therefore, even if a patent for a drug has been granted, the drug cannot be manufactured or sold until MHLW approves it and assigns a drug price. In other words, the patent cannot be commercially utilized until the drug is approved. To compensate patent holders for this commercially inactive period, the patent term extension was introduced in 1988. Under this system, the patent term may be extended by up to five years.
Do other countries have a patent term extension?
Yes, several countries have introduced patent term extension systems. These include the United States, the European Union, Canada, South Korea, Taiwan, China, and Australia. While the specific details of each system vary by country, they commonly allow for an extension of up to five years—except in Canada, where the maximum extension is two years.
Which period is subject to extension?
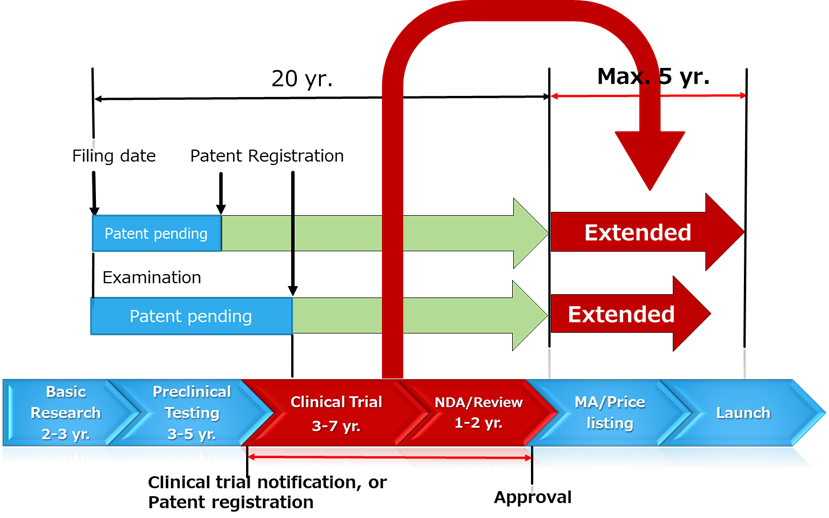
Let’s look at the diagram below to get a clearer understanding about the period subjected to extension. The arrow chart at the bottom shows the schedule for drug development. The red segments labeled “Clinical Trial” and “NDA/Review” represent the period eligible for extension. Up to five years of this period may be added to the original 20-year patent term.
The red segment begins on the date the clinical trial notification is submitted to the MHLW, prior to the start of clinical trials, and ends on the approval date. However, if the patent is still under examination and not yet granted when the clinical trial notification is submitted, the start date of the extension period will be the patent registration date (more precisely, the patent right registration date), rather than the clinical trial notification date.
Under what circumstances can a patent term be extended?
The patent term extension is linked to approval. Upon drug approval, the commercially inactive period in order to obtain that approval is calculated on a daily basis, and the patent term can be extended by up to five years.
In general, there are some Approval categories: “initial approval” for a given active ingredient, and “subsequent approvals,” such as for additional indications, dosage forms, or administration routes. And uniquely to Japan, any approval categories are eligible for patent term extensions, whereas most other countries only allow extensions for the initial approval, or in some cases, only for initial approval and additional indications. Therefore, Japan’s system may be considered more favorable to originator companies compared to those of other countries.
How can a patent term be extended?
To extend a patent term, the patent holder must file a patent term extension application with the Japan Patent Office within three months of drug approval by the MHLW, and no later than the day before the patent expires.
In pharmaceutical companies, the divisions handling MHLW procedures, such as Regulatory Affairs or Development, are often separate from those handling patent office procedures, such as Intellectual Property or Patent departments. Without proper internal communication and coordination, there’s a real risk that the patent term extension application deadline will be missed. To prevent that, we encourage you to share this column with colleagues in Regulatory Affairs and Development so that the patent term extension system is well understood across your organization.
Additionally, caution is needed when a licensee conducts clinical trials and obtains approval. Only the patent holder can file for a patent term extension. Again, if communication between the licensee and the patent holder is lacking, the opportunity to file may be missed. This is especially true when the patent holder is a foreign company, as they may mistakenly believe that extensions are not available for additional dosage form or administration route approvals. To avoid missing such opportunities, it’s important to share accurate information about Japan’s Patent Term Extension. In such cases, we hope you’ll find the English version of this column useful.
When the patent term for an originator drug expires, generic drugs begin to enter the market.
Back in the 1990s, when I began my career, generics were often referred to as “Zoro” in Japan. “Zoro” stems from the Japanese term “zorozoro”, (“to creep in”). But do generics really “creep in”?
Next time, we’ll explore the development and launch process of generic pharmaceuticals. Stay tuned!
Author Profile

Yasuko Tanaka
President & CEO, S-Cube Corporation / Patent Attorney, S-Cube International Patent Firm
Outside Director, Strategic Capital Inc.; Part-time Lecturer, Tokyo University of Agriculture and Technology Graduate School; Technical Advisor for IP-related Litigation
Ms. Tanaka graduated from Chiba University (Biochemistry) in 1990. She has worked in the IP Dept. of Teijin, Pfizer Japan and 3M Japan dealing with global patent prosecution both in English and Japanese, IP strategy/consulting, transactions, and IP education. She resigned from her last company, 3M Japan, in March 2013 to start her own venture “S-Cube Corporation” (IP Business Consultancy) in April. In August, she expanded her firm establishing S-Cube International Patent Firm.