The Path to Clinical Relevance: Moving from Spatial Transcriptomics to Protein Profiling
Spatial biology is a rapidly evolving field that leverages imaging technologies to advance human health. This domain encompasses a broad range of research activities, from whole-transcriptome analysis to clinically meaningful single-protein biomarker assays.
Translational researchers aiming to build clinically useful protein-profiling panels based on insights from whole-transcriptome data face several challenges. These include selecting the appropriate biomarkers for the panel, validating reagents to ensure consistent results, and measuring large numbers of clinical samples to statistically associate findings with clinical outcomes.
Translational spatial biology research enables large-scale quantification of the tissue microenvironment, providing mechanistic, prognostic, and predictive insights that contribute to improved patient care. In this context, protein-level analysis is considered the gold standard for understanding disease mechanisms and evaluating therapeutic responses. A central challenge for researchers is: “How can we design clinically relevant biomarker panels that accurately capture biological complexity while achieving sufficient throughput for clinical research?”
From Discovery to Clinical Value
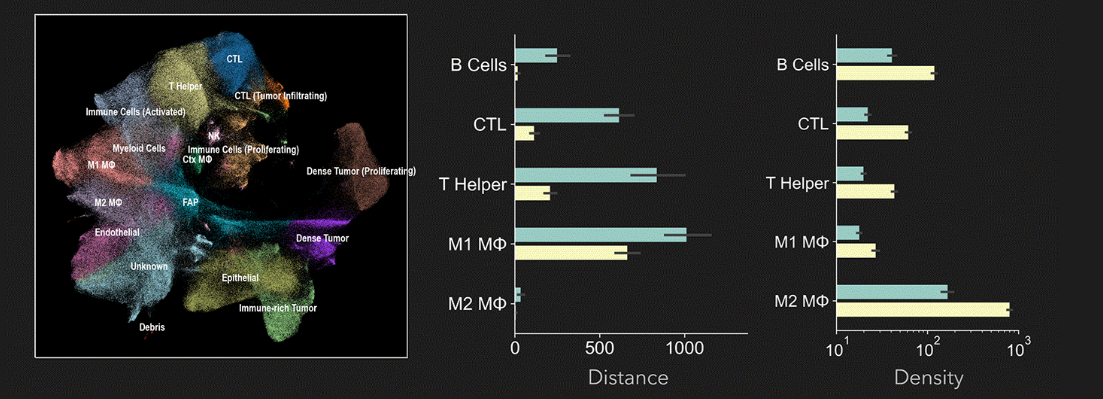
Over the past decade, discovery-stage research using spatial transcriptomics has dramatically expanded the number of microenvironment biomarker candidates with potential clinical utility. Translational research is essential for assessing their relevance.
First, RNA leads are validated at the protein level by focusing on protein expression, which determines cellular phenotype and function. Next, large-scale cohort studies are conducted to relate the microenvironment findings to clinical outcomes.
The general workflow from spatial transcriptomics to multiplex immunofluorescence (multiplex IF) panel testing is as follows:
- Use spatial transcriptomics to generate a shortlist of RNA-based candidate leads within the tissue
- Validate the RNA leads at the protein level using immunohistochemistry (IHC)
- Combine validated protein markers with established markers to build a multiplex IF panel
At this stage, researchers face the question: “What spatial protein-profiling technology offers sufficient throughput and multiplexing to derive clinically meaningful spatial biomarkers backed by statistical power from large-scale cohort studies?”
“Discovery-stage technologies such as transcriptomics have revealed numerous biomarker candidates with potential clinical value. The current challenge is to integrate these promising RNA leads into robust biomarker panels that can be applied in large-scale cohort studies to evaluate clinical utility.”
— Tad George, VP of Biology R&D, RareCyte Inc.
Overcoming the Limitations of Conventional Technologies
In translational spatial biology, it is essential to develop reliable panels with sufficient multiplexing to resolve the tissue microenvironment, as well as to perform high-throughput measurements that provide statistically meaningful clinical insights.
High-throughput protein analysis is indispensable for establishing clinically valuable biomarker panels across large cohorts. However, in practice, the number of markers measurable in a single round often limits throughput.
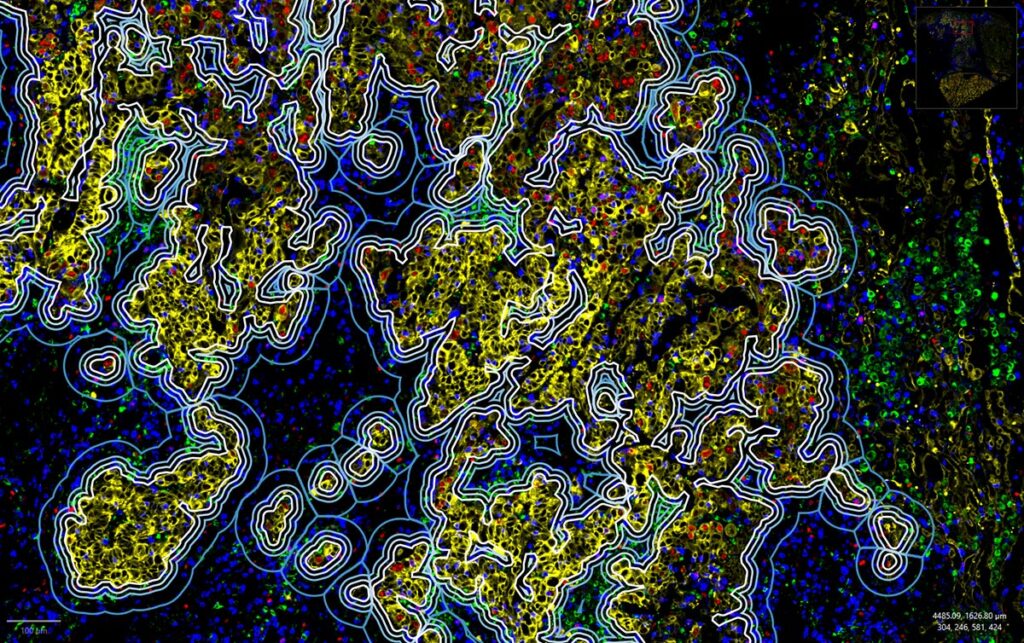
Conventional multiplex IF technologies typically measure only 2–6 markers per round, limiting the depth of analysis. Increasing the number of measurable protein markers expands biological insight and improves data quality.
Orion™ overcomes this limitation by enabling acquisition of 20 channels (18 markers + DNA + autofluorescence) in a single round. When panels exceed a single round, limited cycling on a small number of samples can be used to identify combinations suitable for one-round analysis; however, additional cycling increases time, degradation risk, and reduces throughput. Thus, Orion’s ability to acquire more markers in one round minimizes required cycling, streamlines workflows, and improves data quality.
Regarding the number of plexes needed for clinical research, a study by Dr. Peter Sorger’s group at Harvard University (link) notes that while current clinical-research standards involve 5–6-plex panels, tumor profiling requires at least 10–16 markers.
Challenges in multiplex IF panel design extend beyond antibody selection and include:
- Rigorous validation to ensure each antibody is compatible with the platform and yields accurate, reproducible results
- The ability to produce and validate custom reagents when working with novel targets
- Verification of complete signal removal and preservation of antigenicity when multiple rounds are required
Meeting these requirements is essential for producing high-quality, reproducible data in translational spatial biology studies.
Orion™: A Platform to Bridge the Translational Gap
Orion™ addresses major limitations in spatial biology by enabling simultaneous detection of 20 channels in a single round. This allows efficient transition from RNA-based discovery to protein-level translational research without compromising sample integrity or data quality.
Flexible, High-Quality, and Highly Reliable Panel Development
Orion’s panel-development tools include pre-validated reagents, commercial panels, and labeling kits that make it easy to incorporate custom markers. This significantly shortens panel-development time. Since all antibodies can be stained in a single round, validation becomes simpler and data quality improves.
High Throughput and High Plex for Large-Scale Cohorts
Orion provides the high throughput and high multiplexing capabilities required for whole-slide microenvironment analysis. It processes dozens to hundreds of samples in a short period, generating statistically supported quantitative data linked to clinical outcomes.
Bringing Discovery and Translation Together in One Platform
Orion is the only platform that supports both high-plex discovery panels and single-round translational panels. With 20-channel single-round acquisition and optional cycling for even higher plex, researchers can—for example—perform 51-plex discovery analysis over three rounds and then refine the findings into a clinically focused single-round panel for high-throughput validation, all on a single instrument.
Conclusion
Orion enables high-plex, high-throughput spatial protein analysis across large sample sets, providing strong support for biomarker validation in clinical studies. By connecting discovery-stage spatial transcriptomics with functional validation at the protein level, Orion bridges the gap from biomarker discovery to clinical research, accelerating translation of spatial-biology insights into patient benefit.